Non-sterical stabilization of one-electron-oxidized NiSalen complex by thiophene core†
Abstract
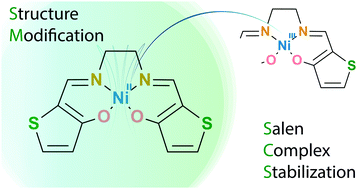
Oxidation of nickel salen complexes results in highly reactive species, undergoing polymerisation within microseconds. As a result, oxidation chemistry of NiSalens is available only for sterically hindered complexes. In the present work, an unexpectedly high stability of a nickel complex with a thiophene-based salen ligand was reported and studied. Based on the electrochemical and spectroscopic studies supported by quantum chemical calculations, a rationale for the stability of oxidized nickel complexes with thiophene-based salen ligands was proposed. The introduction of the thiophene core leads to a depletion of spin density on peripheral carbon atoms of the oxidized complex, which switches off its intermolecular reactivity. Instead of this, the initial ligand-centered radical cation undergoes an intramolecular process, namely intervalence tautomerisation, which leads to a highly stable metal-centered oxidized state.


 Please wait while we load your content...
Please wait while we load your content...