Synthesis of hydroxymethyl analogues of mannostatin A and their evaluation as inhibitors of GH38 α-mannosidases†
Abstract
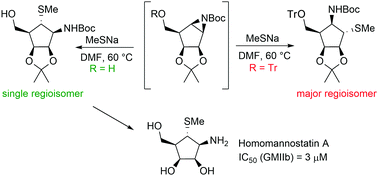
A synthetic approach to hydroxymethyl analogues of mannostatin A starting from L-ribose is described. The key step employed in the synthesis of homomannostatin A was ring-opening of aziridine intermediates with sodium methanethiolate in DMF. Regioselectivity of these openings was investigated by quantum mechanics calculations. The synthesised hydroxymethyl analogues of mannostatin A were evaluated as inhibitors of three different GH38 α-mannosidases: the Golgi (GMIIb) and lysosomal (LManII) α-mannosidases from Drosophila melanogaster, and commercial Jack bean α-mannosidase (JBMan) from Canavalia ensiformis. The tested compounds exhibited inhibitory activity against GMIIb with IC50 values in the range of 3–43 μM resulting in selectivity [IC50(LManII)/IC50(GMIIb)] similar to mannostatin A.


 Please wait while we load your content...
Please wait while we load your content...