Synthesis and evaluation of the antiproliferative activity of new heterylmethylidene derivatives of imidazothiazolotriazinones†
Abstract
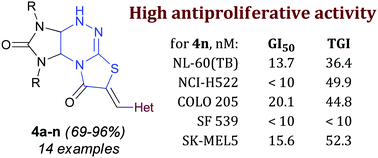
Two novel series of 6-heterylmethylidenetetrahydroimidazo[4,5-e]thiazolo[3,2-b]-1,2,4-triazine-2,7(1H,6H)-diones and 7-heterylmethylidenetetrahydroimidazo[4,5-e]thiazolo[2,3-c]-1,2,4-triazine-2,8(3H,7H)-diones were synthesized by aldol condensation of tetrahydroimidazo[4,5-e]thiazolo[3,2-b]-1,2,4-triazine-2,7(1H,6H)-dione hydrobromides with heteroaromatic aldehydes and subsequent skeletal rearrangement of the thiazolotriazine fragment. The antiproliferative activity of the synthesized compounds was evaluated. Among the derivatives, (Z)-1,3-diethyl-7-[(1H-indol-3-yl)methylidene]-1,3a,4,9a-tetrahydroimidazo[4,5-e]thiazolo[2,3-c]-1,2,4-triazine-2,8(3H,7H)-dione 4n exhibited the highest antiproliferative activity. The GI50 values of the compound against 24 of the 60 cancer cell lines were < 10 nM; against 32 of the 60 cell lines, they were 11.5–63.2 nM; and the GI50 values against the remaining 4 cell lines were 1.33–6.38 μM. Experiments with annexin showed that compound 4n induced apoptosis and necrosis in Jurkat cells (acute T cell leukemia).


 Please wait while we load your content...
Please wait while we load your content...