CoOx/UiO-66 and NiO/UiO-66 heterostructures with UiO-66 frameworks for enhanced oxygen evolution reactions†
Abstract
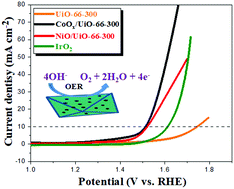
The water oxidation reaction involves a four electron–proton coupled process that is kinetically sluggish and has hindered the widespread application of water-splitting technology. Metal-MOF-coupled heterostructures serve as good OER electrocatalysts due to their endowed MOF frameworks and numerous transition metal active sites at the interface. In this study, a simple ultrasonic impregnation strategy was used to prepare CoOx/UiO-66-300 and NiO/UiO-66-300 heterostructures via the impregnation of Co and Ni ions into UiO-66 followed by roasting in air at 300 °C to obtain heterostructures with UiO-66-retained frameworks as revealed by SEM. The electrochemical results show that the respective impregnation successfully modulated the electronic charge at the interface of the heterostructures, leading to rapid electron transfer that enhanced the OER activity. CoOx/UiO-66-300 and NiO/UiO-66-300 heterostructures showed good oxygen evolution reaction activities of 283 mV and 291.6 mV, respectively, due to the retained UiO-66 framework, while CoOx/UiO-66-550 and NiO/UiO-66-550 heterostructures calcined at 550 °C showed a decrease in OER activities of 332.6 mV and 346.6 mV, respectively, due to the collapsed UiO-66 framework. This work gives insight into the development of heterostructures of MOF-retained frameworks for energy applications.


 Please wait while we load your content...
Please wait while we load your content...