Copper-catalyzed efficient access to 2,4,6-triphenyl pyridines via oxidative decarboxylative coupling of aryl acetic acids with oxime acetates†
Abstract
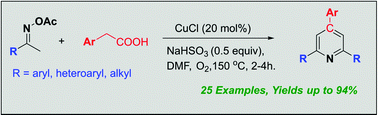
An efficient and concise approach for the synthesis of 2,4,6-triphenyl pyridines has been developed through copper-catalysed oxidative decarboxylative coupling of C(sp3) aryl acetic acids with oxime acetates in DMF at 150 °C under an oxygen atmosphere. Various functional groups were well tolerated and provided the corresponding 2,4,6-triphenyl pyridines in good to excellent yields.


 Please wait while we load your content...
Please wait while we load your content...