Inhibition of non-enzymatic glycation by capsaicin: targeting AGE-induced diabetic complications
Abstract
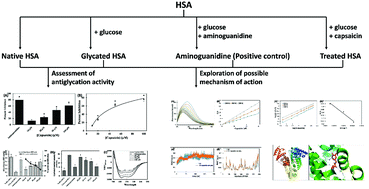
The end products of non-enzymatic glycation are termed advanced glycation end products, i.e., AGEs, which are a diverse heterogenous group of compounds. The highly reactive nature of AGEs makes them one of the major contributors to the complications associated with diabetes. In this study, we explored the antiglycation potential of capsaicin against glucose-induced glycation using human serum albumin (HSA) as the model protein. An array of biophysical and computational tools was employed to decipher the possible mechanism of action. More than 60% inhibition of early glycation products and more than 75% inhibition of advanced glycation end products were found. Improvement in free lysine modification and biochemical markers was recorded by the treatment of capsaicin. Binding studies deciphered that capsaicin exhibited moderate interaction affinity towards HSA and the binning constant was found to be in the order of 105 M−1. The binding between capsaicin and HSA was spontaneous in nature and energetically favorable. Molecular dynamics simulation studies showed that the HSA–capsaicin complex was stable under physiological conditions where RMSD, Rg, RMSF, SASA, and secondary structure of the protein did not alter much over entire simulation duration. The average binding energy for the capsaicin–HSA complex was found to be negative and favored by van der Waals, SASA and electrostatic energy. The findings clearly validate the antiglycation activity of capsaicin with the possible mechanism of action, which could be developed as an inhibitor of glycation.


 Please wait while we load your content...
Please wait while we load your content...