Considerations of the biosynthesis and molecular diversity of tolyporphins
Abstract
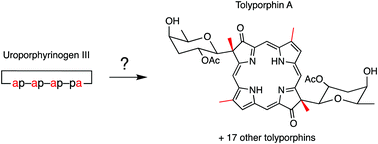
Tolyporphins A–R constitute fundamentally distinct members of the tetrapyrrole pigments of life family. The 18 members present diversity at multiple levels including the chromophore (dioxobacteriochlorin, oxochlorin, porphyrin); composition of the pyrroline substituents (hydroxy, acetoxy, or one of four C-glycosides); and stereochemical configuration of the pyrroline substituents. Eleven of the 18 tolyporphins contain at least one C-glycoside; each C-glycoside has a β,D configuration and lacks a 6′-hydroxy group: 3′,6′-dideoxygalactose (common name abequose), 2′-O-acetyl-3′,6′-dideoxygalactose (2′-O-acetylabequose), 6′-deoxygalactose (D-fucose), or 6′-deoxygulose (antiarose). Rare are such glycosides outside of tolyporphins: (2′-O-acetyl)abequose is reported only in the glycan polymer attached to the cell wall of two strains of Gram-negative bacteria, and antiarose is reported in one bacterial natural product and ∼50 plant cardiac glycosides. Eight of the 18 tolyporphins are bis(C-glycosides), an exceptionally uncommon motif in natural products. The biosynthetic pathways to the family of tolyporphins remain unknown. Regardless of such diversity, each tolyporphin member shares a common pattern of perimeter methyl substituents that coheres with derivation from uroporphyrinogen III, the universal precursor in the established pathway to native tetrapyrroles. Here, transformations required to convert uroporphyrinogen III to all 18 tolyporphins are considered in the context of plausible biosynthetic pathways. Heme d1, perhaps the closest relative (yet still a distant cousin) of tolyporphins, and for which key biosynthetic transformations remain undeciphered, provides a point of reference. Taken together, the work provides the foundation for bioinformatic searching for enzymes associated with the biosynthesis and diversification of tolyporphins.


 Please wait while we load your content...
Please wait while we load your content...