A copper-catalyzed synthesis of aryloxy-tethered symmetrical 1,2,3-triazoles as potential antifungal agents targeting 14 α-demethylase†
Abstract
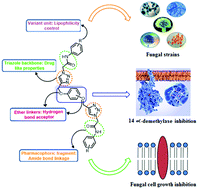
The search for potent therapeutic agents has prompted the design and synthesis of a library of twenty-six aryloxy-tethered and amide-linked symmetrical 1,2,3-triazoles (8a–z) using a copper(I)-catalyzed click chemistry approach. All the synthesized compounds have been screened for their in vitro antifungal activity against four different fungal strains as well as the enzymatic study for the inhibition of 14 α-demethylase enzyme. The bioactivity results show that most of the synthesized compounds were found to be better antifungal agents as compared to Miconazole. Among them, compound 8a showed the most promising antifungal activity against all the tested fungal strains. Furthermore, the enzymatic study reveals that compounds 8i and 8o are the most promising inhibitors of the 14 α-demethylase enzyme. In support of these results, the molecular docking study of the synthesized molecules against the sterol 14 α-demethylase (CYP51) could provide the structural basis for the antifungal activity. These compounds have also been analyzed for the ADME properties.


 Please wait while we load your content...
Please wait while we load your content...