Determination of equilibrium constants of iron(iii)-1,2-dihydroxybenzene complexes and the relationship between calculated iron speciation and degradation of rhodamine B
Abstract
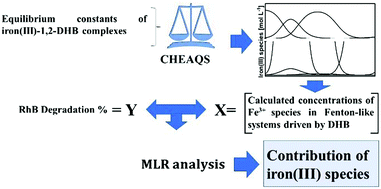
Advanced oxidation processes (AOPs) are chemical systems characterized by the production of hydroxyl radicals and other reactive oxygen species. One of the most important AOP systems are the Fenton and Fenton-like reactions. The main limitation in the reactivity of these systems is the solubility and redox equilibria of Fe3+/Fe+2 species. In this way, the use of iron ligands (redox active or redox inert) is one of the most common ways to modulate the Fenton reaction. These systems are called Chelated Mediated Fenton (CMF) reaction. The 1,2-dihydroxybenzene (1,2-DHB) type ligands are one of the most used redox active compounds to drive a Fenton reaction because these ligands can chelate Fe3+ and reduce it to Fe2+ enhancing de ability of Fenton systems to generate hydroxyl radicals. These systems are called the dihydroxybezene driven Fenton reaction. In this study, the effects of five 1,2-DHBs (substituted in position 4) for driving the Fenton reaction were evaluated. The DHBs utilized were: 3,4-dihydroxybenzoic acid and 3,4-dihydroxybenzonitrile (electron-withdrawing substituents), 4-tert-butylcatechol and 4-methylcatechol (electron-donating substituents) and catechol (without substituent). The degradation of rhodamine B by different Fe3+/H2O2/1,2-DHB systems were determined at pH values between 1 and 9. These values were correlated (by a multivariate model) with the Fe+3 speciation at these pH values. To determine the Fe+3/1,2-DHB complex concentration, the stability constants for the mono, bis and tris complexes were experimentally determined. The log β1, log β2 and log β3 for the assayed 1,2-DHB were 19.30, 27.21, 45.69 for catechol; 19.52, 28.90, 44.66 for 4-methylcatechol; 19.93, 27.16, 44.77 for 4-tert-butylcatechol; 15.57, 23.46, 42.03 for 3,4-dihydroxybenzonitrile and 17.68, 29.79 and 46.27 for 3,4-dihydroxybenzoic acid, respectively. From the multivariate model (R2 = 0.994), it was determined that only Fe+3 aquocomplex, [Fe(OH)2]+ and [FeDHB]+ were significant (p < 0.05) for rhodamine B degradation, which is independent of the nature of the DHB utilized.


 Please wait while we load your content...
Please wait while we load your content...