Uniform heterostructured MnOx/MnCO3/Fe2O3 nanocomposites assembled in an ionic liquid for highly selective oxidation of 5-hydroxymethylfurfural
Abstract
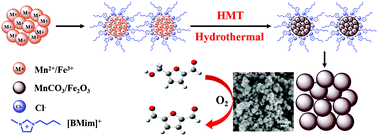
The development of non-precious metal catalysts for highly selective oxidation of the platform chemical 5-hydroxymethylfurfural (HMF) to 2,5-diformylfuran (DFF) remains a challenge. Herein, we prepared uniform heterostructured MnOx, MnCO3, and Fe2O3 (MnOx/MnCO3/Fe2O3) nanocomposites assembled in the ionic liquid 1-butyl-3-methyl-imidazolium chloride ([BMim]Cl) via a hexamethylenetetramine (HMT) assisted hydrothermal process, and these nanocomposites could be used as efficient catalysts for the aerobic oxidation of HMF. Powder X-ray diffraction (XRD), field emission scanning electron microscopy (FE-SEM), transmission electron microscopy (TEM), N2 absorption/desorption, thermogravimetric and differential thermal analysis (TG–DTA), X-ray photoelectron spectroscopy (XPS), inductively coupled plasma atomic emission spectrometry (ICP-AES), Fourier transform infrared (FT-IR) spectroscopy, and H2 temperature programmed reduction (H2-TPR) were carried out to characterize the structural properties of the nanocomposites. In the preparation process, MnCO3/Fe2O3 was initially formed via hydrogen bond and π–π stacking interactions between [BMim]Cl and the metal precursor, where [BMim]Cl was used as a capping agent and HMT not only served as a carbonate source but also acted as a precipitating agent. Thus, a uniform heterostructured MnOx/MnCO3/Fe2O3 nanocomposite with a size distribution of about 60 nm was obtained through calcination at the desired temperature and it exhibited superior catalytic performance with a HMF conversion of 48% and high selectivity (100%) to DFF, which was attributed to the synergistic effect of MnOx, MnCO3, and Fe2O3 in the composite. The Mn/Fe mole ratio and HMT amount could tune the composition of MnOx, MnCO3, and Fe2O3, influence the reduction capability of the prepared nanocomposite via synergisitic effects, and thus have a definite effect on catalytic activity. Additionally, the effects of various conditions were investigated to optimize the reaction conditions. Furthermore, the uniform heterostructured MnOx/MnCO3/Fe2O3 composite could be readily recovered and reused without loss of its catalytic activity and thus is suitable for potential applications.


 Please wait while we load your content...
Please wait while we load your content...