Hydroxylated 3-(pyridin-2-yl)coumarins as radical scavengers with potent lipoxygenase inhibitor activity†
Abstract
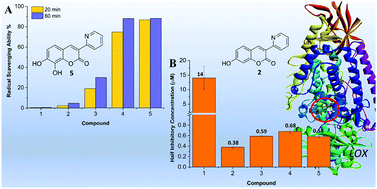
Coumarins comprise a large class of natural-based and synthetic compounds containing a 2Hchromen-2-one ring. Derivatives with hydroxy groups are able to donate a H˙, exhibiting antioxidant activities similar to phenols and quinones. In this work, 3-(pyridin-2-yl)coumarins with and without hydroxy group/s were prepared and their antioxidant ability and inhibition activity towards lipoxygenase enzymes were studied. The studied derivatives have shown potent lipoxygenase activities with IC50 in the μM range, with the radical scavenging properties tuneable according to the number and position of the hydroxy groups. The study of the protonation constants of the 3-(pyridin-2-yl)coumarins, carried out in aqueous solutions, helped in evaluating the absorption and the potential biological properties of the molecules, strongly affected by their protonation state and charge. The antioxidant properties and the way of enzymatic inhibition of the studied 3-(pyridin-2-yl)coumarins are discussed on the basis of the calculated specific thermochemical descriptors and molecular docking.


 Please wait while we load your content...
Please wait while we load your content...