Understanding the molecular mechanism of the stereoselective conversion of N-trialkylsilyloxy nitronates into bicyclic isoxazoline derivatives†
Abstract
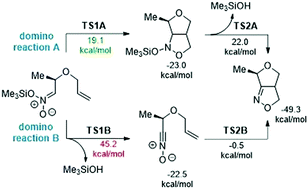
The conversion of N-trialkylsilyloxy nitronates into bicyclic isoxazoline derivatives has been explored using the density functional theory (DFT) method within the context of molecular electron density theory (MEDT) at the ωB97XD(PCM)/6-311G(d,p) theoretical level. The obtained results clearly indicated that this reaction proceeds via a domino process, including two pseudocyclic elemental reactions: nitronate-[3+2] cycloaddition and the elimination of trimethylsilanol. An exploration of the electron localization function (ELF) of the most significant points located along the intrinsic reaction coordinate (IRC) profile elucidates the molecular mechanism of the studied reactions. The bonding electron density changes along the reaction paths reveal that the analysed reactions take place via an asynchronous one-step mechanism. The first reaction of the domino process is characterized by six different phases associated with the formation of two C–C and C–O single bonds, while there are five different topological phases related to the formation of one O–H single bond and one N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond in the second reaction of this domino process.
C double bond in the second reaction of this domino process.


 Please wait while we load your content...
Please wait while we load your content...