ESIPT on/off switching and crystallization-enhanced emission properties of new design phenol-pyrazole modified cyclotriphosphazenes†
Abstract
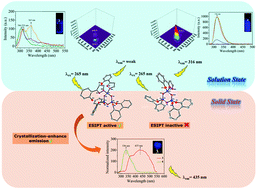
A nucleophilic substitution reaction of N3P3Cl2(2,2′-dioxybiphenyl)2 (1) with 2-(1H-pyrazol-3-yl) phenol (2) was performed in order to synthesize molecules that may exhibit different photophysical properties on the cyclotriphosphazene scaffold. Bis-geminal phenol-pyrazole (3) and mono-spiro phenoxy-pyrazole (4) cyclotriphosphazene derivatives were obtained as a result of an effective single substitution reaction. The structures of the new cyclotriphosphazene derivatives (3 and 4) were illuminated by various spectroscopic techniques such as mass spectrometry (MALDI-TOF), 1H, 31P NMR, and elemental analysis, and also confirmed by single crystal X-ray diffraction. After the structural analysis, their photophysical properties in solution and the solid phase were examined by UV-Vis absorption and fluorescence spectroscopies. At the same time, the functionality of the excited state intramolecular proton transfer (ESIPT) mechanism and crystallization-enhanced emission (CEE), which are an important point, and their relationship with different substitution modes on the cyclotriphosphazene ring were investigated by steady-state fluorescence, time resolved-fluorescence and 3-D fluorescence measurements in solution and the crystalline state. The proton transfer properties of compounds 3 and 4 can lead to highly efficient ESIPT or inhibited-ESIPT and efficient crystallization-enhanced emission (CEE) via changing of the substitution mode. The phenol-pyrazole modified cyclotriphosphazenes (3 and 4) are the first examples where the ESIPT mechanism is active and inactive.


 Please wait while we load your content...
Please wait while we load your content...