Abstract
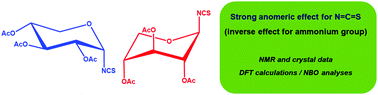
This work is aimed at providing a systematic and comprehensive analysis of the anomeric effect exerted by the isothiocyanato (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S) group taking the xylopyranose ring as a scaffold because enhanced effects have been detected in that ring-bearing halogen and pseudohalogen substituents. To this end, both α- and β-anomers of tri-O-acetoxypyranosyl-D-xylose have been prepared and thoroughly characterized. The axial preference of the isothiocyanato group reflects a dominant anomeric effect, whose origin could be determined through DFT calculations with identification of the stereoelectronic interactions as revealed by Natural Bond Orbital (NBO) analysis. In this way, this study expands the repertoire of groups biasing the conformation and reactivity of monosaccharides and related heterocycles.
S) group taking the xylopyranose ring as a scaffold because enhanced effects have been detected in that ring-bearing halogen and pseudohalogen substituents. To this end, both α- and β-anomers of tri-O-acetoxypyranosyl-D-xylose have been prepared and thoroughly characterized. The axial preference of the isothiocyanato group reflects a dominant anomeric effect, whose origin could be determined through DFT calculations with identification of the stereoelectronic interactions as revealed by Natural Bond Orbital (NBO) analysis. In this way, this study expands the repertoire of groups biasing the conformation and reactivity of monosaccharides and related heterocycles.


 Please wait while we load your content...
Please wait while we load your content...