Impact of the phenolic O–H vs. C-ring C–H bond cleavage on the antioxidant potency of dihydrokaempferol†
Abstract
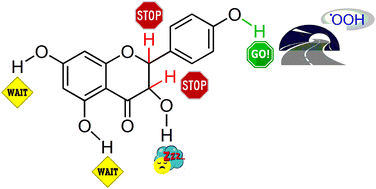
Kinetics and thermodynamics of the phenolic O–H and the C-ring C–H bond breaking in dihydrokaempferol, a natural flavonoid compound, was investigated using a density functional based approach. The rate constants calculated using the variational transition state theory with multidimensional tunneling corrections indicate 4′-OH phenolic hydrogen as operative in the inactivation of a hydroperoxyl radical (˙OOH). The proton coupled electron transfer (PCET) mechanism appears as preferred in O–H bond breaking by ˙OOH, while the hydrogen atom transfer (HAT) mechanism is involved in the C–H bond cleavage. Comparison of the antioxidant potency of dihydrokaempferol vs. kaempferol proves the superiority of the latter and the negligible role of the C-ring hydrogens of dihydrokaempferol in radical scavenging. The obtained results confirm the traditional view that O–H groups of polyphenols are responsible for radical inactivation contrary to recent proposals that the aliphatic C-ring hydrogens also contribute to this activity.


 Please wait while we load your content...
Please wait while we load your content...