Mechanistic insights into the palladium-catalyzed hydroaminocarbonylation of alkenes†
Abstract
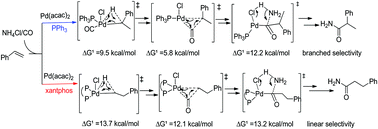
DFT calculations have been conducted on Pd-catalyzed regioselective hydroaminocarbonylation of alkenes. The favored path I consists of styrene insertion, CO insertion, and nucleophilic attack, which is the rate determining step. In the PPh3-assisted system, the conjugation between the benzylic units and the acyl group stabilizes the branched transition state in the nucleophilic attack leading to the branched selectivity. On the other hand, in the xantphos-assisted system, the steric repulsion between the substrate and the ligand leads to a higher barrier for the branched transition state; therefore, linear selectivity is favored which is in line with the experimental observation. The analysis of the electronic and steric effects of ligands on the selectivity helps to develop a catalyst for hydroaminocarbonylation of alkenes.


 Please wait while we load your content...
Please wait while we load your content...