Manganese(ii) Schiff-base-mediated reversible deactivation controlled radical polymerization of vinyl acetate†
Abstract
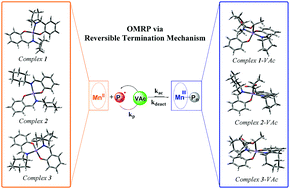
A series of new manganese(II) complexes were prepared with three bidentate Schiff-base ligands derived by condensation of salicylaldehyde and different cycloalkylamines (cycloalkyl = cyclopentyl, cyclohexyl, and cycloheptyl): [Mn(CyPen-Salicyl)2] (1), [Mn(CyHex-Salicyl)2] (2), and [Mn(CyHep-Salicyl)2] (3). The complexes 1–3 were characterized by FTIR, UV-vis and EPR spectroscopy, elemental analysis, cyclic voltammetry, and computational methods. The manganese(II) complexes (1–3) were used as controlling agents for the polymerization of vinyl acetate (VAc) initiated by AIBN according to an organometallic-mediated radical polymerization (OMRP) mechanism. The VAc polymerization mediated by the Mn complexes suggests that the control level can be slightly tuned through substitution of the cycloalkyl group on the Schiff-base ligand. Kinetics studies and computational investigations support an RT mechanism and a tailorable Mn complex reactivity mainly altered by steric factors of the Schiff-base ligands. The calculated thermodynamic parameters agree with the mediating ability of these complexes, since the degree of polymerization control decreases with increasing steric hindrance of the Schiff-base ligands, as well as with increasing ΔG values for the formation of dormant species.


 Please wait while we load your content...
Please wait while we load your content...