BiCl3-Facilitated removal of methoxymethyl-ether/ester derivatives and DFT study of –O–C–O– bond cleavage†
Abstract
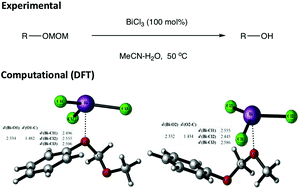
A simple method for the cleavage of methoxymethyl (MOM)-ether and ester derivatives using bismuth trichloride (BiCl3) is described. The alkyl, alkenyl, alkynyl, benzyl and anthracene MOM ether derivatives, as well as MOM esters of both aliphatic and aromatic carboxylic acids, were deprotected in good yields. To better understand the molecular roles of BiCl3 and water for MOM cleavage, two possible binding pathways were investigated using the density functional theory (DFT) method. The theoretical results indicate the differential initial binding site preferences of phenolic and alcoholic MOM substrates to the Bi atom and suggest that water plays a key role in facilitating the cleavage of the MOM group.


 Please wait while we load your content...
Please wait while we load your content...