Enhanced electrocatalytic H2S splitting on a multiwalled carbon nanotubes-graphene oxide nanocomposite†
Abstract
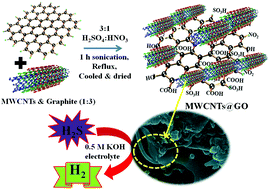
A non-precious graphene oxide (GO) based oxidized multiwalled carbon nanotubes (MWCNTs) metal-free electrocatalytic system was fabricated using a chemical method and was further used for the oxidation of hydrogen sulphide (H2S) to H2. The system demonstrated surprising performance, attributed to the low onset potential in addition to the superior stability. Herein, heteroatoms including nitrogen, sulphur, and oxygen acted as promotors between the MWCNTs and GO for the hydrogen sulphide oxidation reaction. The electrochemical measurements indicated that the composite has a superior current density of 98.20 mA cm−2 with better stability towards the H2S oxidation reaction. This enhancement will subsequently increase the current density for H2 generation from H2S at an onset potential of −0.5 V versus a saturated calomel electrode (SCE). Furthermore, compared with the initiation barrier on different surfaces, it was observed that the presence of S atoms can facilitate the first S–H bond dissociation and drive the second dissociation of S–H species.


 Please wait while we load your content...
Please wait while we load your content...