Iodine-catalyzed sulfuration of isoquinolin-1(2H)-ones applying ethyl sulfinates†
Abstract
An efficient sulfuration of isoquinolin-1(2H)-ones at the C-4 position is reported by employing ethyl sulfinates, and the corresponding products are obtained in moderate to excellent yields in the presence of iodine. This synthetic strategy provides a range of thioether-isoquinolin-1(2H)-ones while tolerating a number of functional groups on the isoquinoline nitrogen atom and benzene ring. In addition, pyridin-2(1H)-one is also reacted smoothly and afforded the corresponding thioether product in moderate yield. A plausible mechanism is suggested based on the preliminary mechanistic studies.
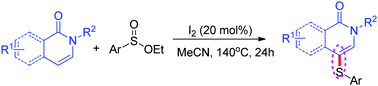


 Please wait while we load your content...
Please wait while we load your content...