Benign synthesis of fused-thiazoles with enone-based natural products and drugs for lead discovery†
Abstract
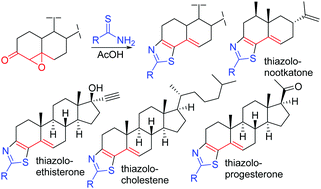
In an effort to synthesize a library of bioactive molecules, we present an efficient synthesis of fused-thiazole derivatives of natural products and approved drugs by using an environmentally usable solvent, acetic acid, and without any external reagent. Cholestenone, ethisterone, progesterone, and nootkatone-derived epoxyketones have been utilized to synthesize 50 novel compounds. The plausible mechanism of the reaction has been determined by theoretical calculation using M06-2X/6-31+G(d,p). These novel molecules have been tested against cancer cell lines and pathogenic bacterial strains. Several ethisterone-based fused-thiazole compounds are found to be potent growth inhibitors of cancer cell lines at submicromolar concentrations.


 Please wait while we load your content...
Please wait while we load your content...