Enhancement of propane combustion activity over CoOx catalysts by introducing C2–C5 diols†
Abstract
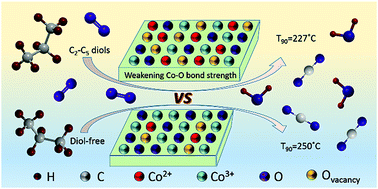
A series of nanocrystalline CoOx catalysts were synthesized by a citric acid complexation strategy. The effects of C2–C5 diols (including ethylene glycol, 1,3-propanediol, 1,4-butanediol, and 1,5-pentadiol) on catalyst structure and propane combustion performance were investigated. The results showed that the structure of CoOx transformed from pure Co3O4 to mixed-phase CoO–Co3O4 with a larger surface area and smaller crystallite size after the introduction of diol compounds. Importantly, diol compounds effectively weaken the Co–O bond strength in nanocrystalline CoOx and enhance the activity of oxygen species, thus effectively degrading propane. Due to the weakest Co–O bond strength, excellent oxygen species activity, abundant oxygen vacancies, and the strongest redox ability, the catalyst prepared by adding 1,3-propanediol (Co-PDO) exhibited the best catalytic activity (T90 = 227 °C). Moreover, the increase in pore size, surface Co2+ and oxygen vacancy content of Co-PDO in the 40 h stability test can compensate for the loss of activity caused by the decrease in specific surface area, thereby maintaining stable catalytic activity.


 Please wait while we load your content...
Please wait while we load your content...