A relativistic DFT probe for small-molecule activation mediated by low-valent uranium metallocenes†
Abstract
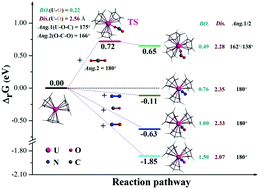
Due to the powerful reducing ability of the metal active site, low-valent uranium complexes show remarkable performance in activating thermodynamically stable and kinetically inert small molecules. In this work, experimentally known uranium metallocenes [Cp3U]z (z = 0 and +1) were intensively studied using relativistic density functional theory for their activation of small molecules (X = CO2, N2, CO and NO). Regardless of whether z = 0 or +1, a weak double bond was realized between uranium and NO, which is in agreement with a previous study; a weak single bond with the dative nature was assigned to other small-molecule uranium adducts. A general order for the uranium–small molecule bond strength, NO > CO > N2 > CO2, has been built, the trend being exactly the same as the computed reduction potentials. These are corroborated by energetics and experimental results that (i) analogues of the first three [Cp3U(X)] adducts were crystallographically identified but the one of [Cp3U(CO2)] was not, and (ii) much higher X gas pressure was required to synthesize [Cp3U(N2)] than for [Cp3U(CO)]. Additionally, the experimentally inaccessible CO2 adduct was rationalized using thermodynamic and kinetic calculations as well as geometric/electronic properties and bonding analyses.


 Please wait while we load your content...
Please wait while we load your content...