Multifunctional glycerol/citric acid crosslinked polymer hydrophilic gel with absorptive and reducing properties†
Abstract
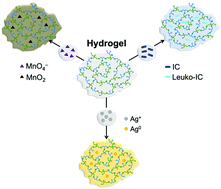
This work describes a hydrophilic polymeric gel with satisfactory water absorption (1.4 gH2O g−1gel at pH = 10) and reducing capacity prepared from the reaction of glycerol oleate esters with citric acid. Elemental analysis and FTIR spectroscopy suggest that the oleate portion of the starting material is not present in the final composition of the isolated gel. Nevertheless, it plays a vital role in the reaction while assisting the formation of the crosslinked polymer composed by glycerol and citric acid in a 1 : 1 molar ratio. This gel allows for the simultaneous adsorption/reduction of dark-blue indigo carmine dye in aqueous medium endorsing its dual role in this process. The reducing capacity of the gel was also confirmed through the reactivity towards permanganate (MnO4−) ions, leading to the formation of MnO2, whose crystalline phase was confirmed by PXRD. Citrate present in the structure of the gel matrix is key to justify its reducing behavior. This property opens new avenues to produce gels bearing noble metal nanoparticles, and we demonstrate this approach via adsorption/reduction of Ag+ ions in aqueous solution that yields a gel containing Ag nanoparticles with potential biomedical applications. This hybrid material was fully characterized by UV-Vis, SEM, and PXRD analyses indicating the formation of highly dispersed Ag0 nanoparticles with an average diameter of 20 nm.


 Please wait while we load your content...
Please wait while we load your content...