Molecular diversity of TEMPO-mediated cycloaddition of ketohydrazones and 3-phenacylideneoxindoles†
Abstract
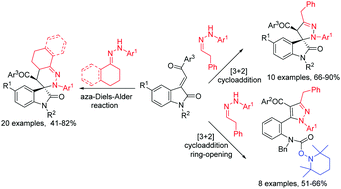
TEMPO promoted cycloaddition reaction of different kinds of ketohydrazones or aldohydrazones with 3-phenacylideneoxindoles showed very interesting molecular diversity according to the chemical structures and reaction conditions. The reactions with 1-(3,4-dihydronaphthalen-1-ylidene)-2-arylhydrazines or 1-cyclohexylidene-2-arylhydrazines in acetonitrile at 80 °C afforded functionalized spiro[benzo[h]cinnoline-3,3′-indolines] or spiro[cinnoline-3,3′-indolines] via aza-Diels–Alder reaction of the in situ generated 1,2-diaza-1,3-diene. The reactions with 2-(2-phenylethylidene)-1-arylhydrazines in dioxane afforded functionalized spiro[indoline-3,3′-pyrazoles] though a [3+2] cycloaddition reaction of the in situ generated 1,3-dipolar azomethine imine. On the other hand, a similar reaction in acetonitrile unusually resulted in pyrazole derivatives via sequential [3+2] cycloaddition and a ring-opening process.


 Please wait while we load your content...
Please wait while we load your content...