A transient non-covalent hydrogel by a supramolecular gelator with dynamic covalent bonds†
Abstract
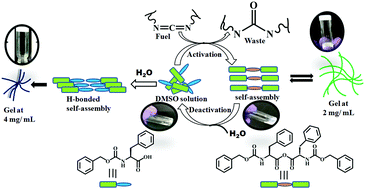
Nature operates out of equilibrium, which needs a continuous input of energy. However, on the removal of the energy source, the system returns to its thermodynamically stable building blocks, resulting in an aggregation-to-nonaggregation transition. The control of this system is governed by kinetics. Herein, we have alleviated that system using a supramolecular gelator with dynamic covalent bonds. In the aqueous solution of benzyloxycarbonyl-L-phenylalanine (ZF), equilibrium self-assembly and gelation take place at 4 mg mL−1; however, on the addition of 1-ethyl-3-(3-(dimethylamino)propyl)carbodiimide (EDC), non-equilibrium hydrogels are formed at 2 mg mL−1 due to anhydride formation and self-assembly. However, with time, the hydrolysis of anhydride results in a gel-to-sol transition. The dynamic covalent bond formation and rupture have programmed the dissipative, transient supramolecular hydrogels from ZF with a high degree of control over the self-assembly lifetime. The system is not fully dissipative as it does not spontaneously oscillate between two states, but needs fuel to undergo a transition. The refueling of the system with EDC helps to access multiple cycles.


 Please wait while we load your content...
Please wait while we load your content...