Synthesis and characterization of new Na+ complexes of N-benzyl cyclic peptoids and their role in the ring opening polymerization of l-lactide†
Abstract
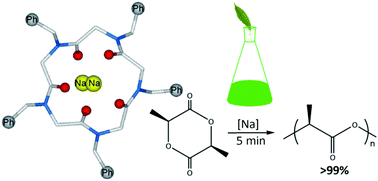
Cyclic peptoids are biocompatible/biodegradable cyclooligomers constituted by N-substituted glycines showing high binding constants with the first group alkali metals (Ka ∼ 106 for Na+, Li+ and K+) in organic solvents. Three new metallated species bearing N-benzyl groups as side chains (a cyclic pentamer, [1·2Na]2+, and two cyclooctamer [3·Na]+ and [3·2Na]2+ peptoids) were synthesized together with the known N-perbenzylated sodiated cyclohexamer [2·Na]+ and [2·2Na]2+. Na+ complexes were investigated by means of spectroscopic (NMR), computational (DFT) and X-ray crystallographic studies. The first application of these complexes as catalysts in the ring opening polymerization (ROP) of L-lactide (L-LA) was examined. Our studies suggest that, for the intrinsic properties of the catalytic site (revealed by steric topographic maps) the homooligomeric pentamer complex [1·2Na]2+ is the most active member of the cyclooligomeric family.


 Please wait while we load your content...
Please wait while we load your content...