Molecular iodine mediated oxidative cleavage of the C–N bond of aryl and heteroaryl (dimethylamino)methyl groups into aldehydes†
Abstract
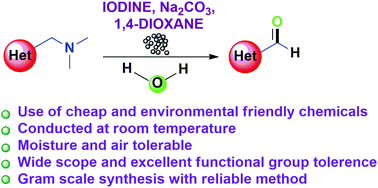
The oxidative cleavage of the C–N bond of aryl and heteroaryl (dimethylamino)methyl groups is achieved by employing molecular iodine as a mild oxidizing agent under ambient conditions in the presence of a mild base. The important reaction of C3 formylation of free NH and substituted indoles containing various substituents is accomplished from the corresponding Mannich bases. This methodology can also be extended for the synthesis of aryl and other heteroaryl aldehydes and ketones. Furthermore, the usefulness of the method is successfully demonstrated on a gram scale.


 Please wait while we load your content...
Please wait while we load your content...