Ala–Ala dipeptides with a semi-perfluoroalkyl chain: chirality driven molecular packing difference and self-assembly driven chiral transfer†
Abstract
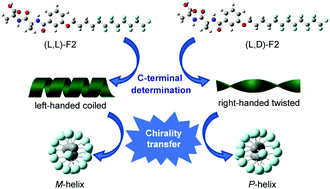
Four Ala–Ala dipeptides with a semi-perfluoroalkyl chain were synthesized. The intermolecular hydrogen bonding among amide groups and carboxyl groups as well as the hydrophobic association of semi-perfluoroalkyl chains drove the self-assembly of dipeptides. Homochiral dipeptides were able to self-assemble into coiled nanoribbons in a mixed solvent of DMSO/H2O (5/5, v/v), while entangled twisted nanofibers formed for heterochiral ones in a mixed solvent of DMSO/H2O (4/6, v/v). The handedness of self-assemblies and the stacking chirality of phenylene groups were controlled by the chirality of the alanine residue at the C-terminal. The vibration circular dichroism investigation indicated that the helicity of the semi-perfluoroalkyl chain was controlled by the handedness of dipeptide self-assemblies. The X-ray diffraction study showed that homochiral and heterochiral dipeptides underwent distinct molecular packing during the self-assembly. Our results clearly demonstrated that, through supramolecular self-assembly, the chirality transferred from the amino acid building block to the self-assemblies and eventually to the semi-perfluoroalkyl chain.


 Please wait while we load your content...
Please wait while we load your content...