Stabilization of caesium ions by simple organic molecules: crystal structures of Cs(OXL) (OXL = oxalurate anion) and the CsOH/cyanuric acid co-crystal Cs3(CYH3)4(OH)3 (CYH3 = cyanuric acid)†
Abstract
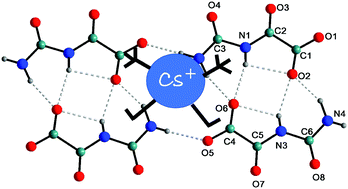
The reaction in water between CsOH and parabanic acid (PBH2) leads to the formation of the caesium salt of the oxalurate anion Cs(OXL), while the reaction with cyanuric acid (CYH3) leads to the formation of the CsOH co-crystal of cyanuric acid Cs3(CYH3)4(OH)3. The X-ray crystal structures of these compounds show that both the organic moieties OXL and CYH3 form robust homomeric ribbons based on strong and articulated N–H⋯O hydrogen bonds. The stabilization of the Cs+ ions can occur regardless of whether the ribbon of organic units is negatively charged or neutral. In Cs(OXL), each cation displays nine-fold coordination with Cs–O distances in the range of 2.975(3)–3.601(4) Å; in Cs3(CYH3)4(OH)3, two of the Cs+ cations (Cs1 and Cs2) display a nine-fold coordination with Cs–O distances in the range of 3.007(9)–3.823(13) Å and one (Cs3) is ten-fold coordinated with Cs–O distances in the range of 3.161(14)–3.653(17) Å. The molecular electrostatic potential maps of OXL and di-OXL anions have been reported and discussed.


 Please wait while we load your content...
Please wait while we load your content...