Enantiopure methoxetamine stereoisomers: chiral resolution, conformational analysis, UV-circular dichroism spectroscopy and electronic circular dichroism†
Abstract
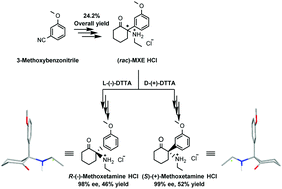
The chiral molecule, methoxetamine (MXE), demonstrated promising biological effects in management of treatment-resistant depression patients. To satisfy the need for a method capable of providing gram quantities of each enantiopure stereoisomer of MXE to enable advanced biological studies of enantiomers, a protocol was developed to access gram scale quantities of (R)- and (S)-MXE in the form of pharmaceutically acceptable HCl salts employing L-(−)-DTTA and D-(+)-DTTA ((−)-O,O′-di-p-toluoyl-L-tartaric acid and (+)-O,O′-di-p-toluoyl-D-tartaric acid, respectively) as two chiral resolving agents. In contrast to ketamine, the measured specific optical rotation and conformational analysis indicated that the most abundant conformers possess a common axial-methoxyphenyl conformation responsible for the conserved direction of optical rotation in both free base and HCl salt forms of the MXE stereoisomers. Finally, the absolute configuration was unambiguously assigned through matching experimental and calculated ECD spectra. This report offers a gateway to obtain gram scale amounts of enantiopure MXE stereoisomers needed to advance the current knowledge on MXE biology.


 Please wait while we load your content...
Please wait while we load your content...