Improved electrochemical performance of lanthanum-modified Na3V2(PO4)3/C cathode materials for sodium-ion batteries
Abstract
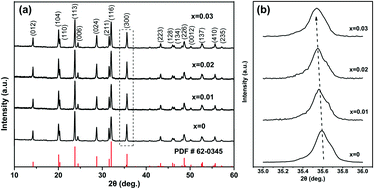
A series of lanthanum-doped Na3V2−xLax(PO4)3/C (0 ≤ x ≤ 0.03) composites have been fabricated via a simple sol–gel approach. The influences of La3+ substitution on the structure, morphology and electrochemical properties are systematically studied. Rietveld refinement is applied to explore the actual substitution site of La, which proves that La3+ ions successfully occupy the octahedral V sites. It is suggested that appropriate La doping will reduce the particle size and enhance the reversible capacity and rate capability. Among all the samples, the as-prepared Na3V1.99La0.01(PO4)3/C compound shows the highest electrochemical capability. It delivers a high initial specific capacity of 111.3 mA h g−1 at 0.2C and maintains a capacity of 108.1 mA h g−1 over 100 cycles, exhibiting much better electrochemical performance than the original Na3V2(PO4)3/C. In addition, cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) experiments suggest that low-level La doping can greatly decrease the polarization and increase electrical conductivity with fast reaction kinetics and a higher Na+ diffusion coefficient. The study presented here will provide considerable insights into rare earth element-doping tactics, which will facilitate the commercialization of sodium-ion batteries.


 Please wait while we load your content...
Please wait while we load your content...