Structural spectroscopic study of enantiomerically pure synthetic cathinones and their major metabolites†
Abstract
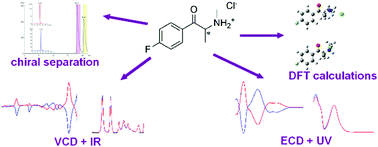
New psychoactive substances (NPSs) have become a popular alternative to illicit drugs of abuse. However, to determine their metabolic pathways in the human organism, a detailed knowledge of their structure is crucial. Here, we present a comprehensive spectroscopic structural study of synthetic cathinones (clephedrone, flephedrone, and brephedrone) and their major human metabolites, desmethyl derivatives. Chiral high-performance liquid chromatography was utilized to obtain the individual enantiomers of the parent synthetic cathinones and their assumed major metabolites synthesized de novo. The developed chromatographic method made it possible to obtain the target optically pure substances on a multimilligram scale. Electronic and vibrational circular dichroism, combined with infrared and ultraviolet spectroscopy and supported by DFT calculations, were used to determine their absolute configuration and the chiroptical methods to elucidate their molecular structure in detail. Two stable conformers of each substance were found in aqueous solution. Their relative abundances were estimated based on the Boltzmann distribution and the population weighted spectra were obtained. Very good agreement was achieved between the experimental and simulated spectra, enabling the 3D structures of the studied substances to be determined in aqueous solution.


 Please wait while we load your content...
Please wait while we load your content...