Reductive amination of bio-based 2-hydroxytetrahydropyran to 5-Amino-1-pentanol over nano-Ni–Al2O3 catalysts†
Abstract
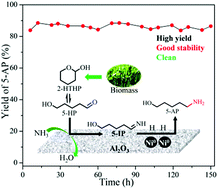
The synthesis of useful amines from bio-based carbonyl compounds is highly desired owing to their mild reaction conditions and green sustainability. The reductive amination of bio-furfural-derived 2-hydroxytetrahydropyran (2-HTHP) to high-value-added 5-Amino-1-pentanol (5-AP) was carried out over efficient Ni–Al2O3 catalysts prepared by a co-precipitation method. Among the Ni–Al2O3 catalysts with different Ni loadings (0–100 wt%) tested, the 50Ni–Al2O3 catalyst exhibited the highest 5-AP yield of 91.3% under mild conditions of 60 °C and 2 MPa H2. This catalyst also presented good stability during a 150 h time-on-stream without appreciable deactivation. Characterization results showed that the 50Ni–Al2O3 catalyst exhibited small Ni0 nanoparticles (5.5 nm), a high reduction degree (up to 95%), and a large amount of strong Lewis acid sites. The cooperative catalysis of the strong Lewis acid sites and highly dispersed metallic Ni sites is suggested to play an important role in achieving high efficiency in 2-HTHP reductive amination.


 Please wait while we load your content...
Please wait while we load your content...