Tetrakis-(N-methyl-4-pyridinium)-porphyrin as a diamagnetic chemical exchange saturation transfer (diaCEST) MRI contrast agent†
Abstract
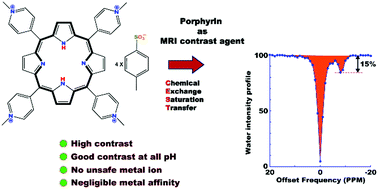
The easily synthesizable highly water-soluble porphyrin derivative tetrakis-(N-methyl-4-pyridinium)-porphyrin (TmPyP) is proposed as a diaCEST contrast agent that efficiently works in a wide range of pH across the physiological pH of 7.4 at 37 °C. The pH invariance of CEST efficiency is orchestrated by the presence of charge balancing counter anions that make the exchange rate constant of the labile inner core amine protons of TmPyP nearly independent of pH in the important physiological range of 6.6 to 8.3. Moreover, the diatropic ring current within the ring makes the labile protons to appear a convenient ∼8 ppm upfield with respect to the bulk water NMR chemical shift. Consequently, TmPyP shows a nearly pH-independent average CEST contrast of 18% in the important pH range in which most human body fluids are reported. With the inherent safety tag of porphyrin due to the absence of a metal center and very low affinity towards metal binding, good water solubility at the acidic pH of 6.5 and lower that is necessary for excretion, pH-independent high contrast efficiency around a physiological pH, and TmPyP is a potential diaCEST agent suitable for in vivo applications.


 Please wait while we load your content...
Please wait while we load your content...