Synthesis of 2′-(1,2,3-triazoyl)-acetophenones: molecular docking and inhibition of in vitro monoamine oxidase activity†
Abstract
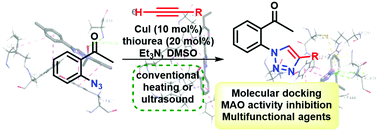
We describe our results for the synthesis of 2′-(1,2,3-triazoyl)-acetophenones by copper catalyzed azide–alkyne cycloaddition of 2′-azidoacetophenone with alkynes. The corresponding 2′-(1,2,3-triazoyl)-acetophenones were obtained in moderate to excellent yields and according to our experiments, it was observed that ultrasound accelerates the reaction compared to conventional heating. In addition, the obtained compounds were screened by molecular docking for their possible inhibitory effect on the activity of monoamine oxidase (MAO), an enzyme responsible for the degradation of monoamine neurotransmitters. Three compounds were selected and presented an inhibitory effect on both MAO-A and B isoform activities in vitro. These new MAO inhibitors may be of interest to treat monoamine deficit related-neuropsychiatric disorders, such as depression and Parkinson's disease.


 Please wait while we load your content...
Please wait while we load your content...