Structure–activity relationship analysis of a series of nonsteroidal analogues as androgen receptor antagonists†
Abstract
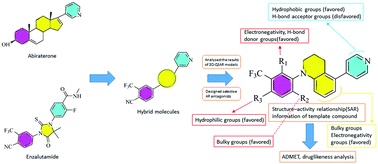
Prostate cancer is the second leading cause of cancer death among men. Nowadays the main therapeutic strategy of prostate cancer is using the androgen receptor (AR) antagonists. However, their anti-cancer properties still need to be improved and a novel AR antagonist should be designed for patients showing different levels of drug resistance after a period of treatment. In this study, we used computer-aided drug design, which is a power tool for drug design and development, successfully designing several new AR antagonists with high anti-cancer activity. We successfully built a three-dimensional quantitative structure–activity relationship (3D-QSAR) model for the AR antagonists through the comparative molecular field analysis (CoMFA) and comparative molecular similarity indices analysis (CoMSIA) of the reported experimental data. The optimal prediction models (Q2 = 0.672 and 0.663, R2 = 0.992 and 0.994, rpred2 = 0.745 and 0.868 for CoMFA and CoMSIA) were obtained. These results reflected that the constructed 3D-QSAR models were stable and robust with predictive ability. Based on the results of the 3D-QSAR, we found that the negative charge groups at the R1 position, the bulky substituents at the R2 position, and the hydrophilic groups at the R3 position all can effectively improve the anti-cancer properties of AR antagonists. Based on these clues, several new AR antagonists were designed with high activity of anti-prostate cancer (pIC50 7.168–7.324 for CoMFA and 7.197–7.422 for CoMSIA). Furthermore, for the novel AR antagonists, their ADMET properties and drug likeness were also analysed. The results show that these novel AR antagonists also have reasonable pharmacokinetics and drug-like properties. Our results can provide important references for the design and development of highly effective AR antagonists.


 Please wait while we load your content...
Please wait while we load your content...