Solid state emissive azo-Schiff base ligands and their Zn(ii) complexes: acidochromism and photoswitching behaviour†
Abstract
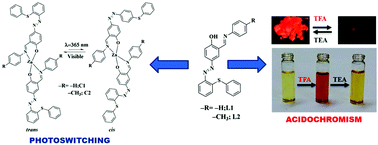
The synthesis of Zn(II) complexes (C1–C2) based on azo-Schiff base ligands 2-((E)-(phenyl-imino)methyl)-4-((E)-(2-(phenylthio)phenyl)diazenyl)phenol (L1) and 4-((E)-(2-(phenyl-thio)phenyl)diazenyl)-2-((E)-(p-tolylimino)methyl)phenol (L2) has been described. These have been thoroughly characterized by spectroscopic studies (IR, 1H, 13C, ESI-MS, electronic absorption, emission) and the structures of C1 and C2 have been determined by X-ray single crystal analyses. The ligands L1 and L2 exhibit reversible acid/base induced “ON–OFF–ON” switching in solution and solid state. Upon exposure to UV light (λ, 365 nm) C1 and C2 display cis–trans photoisomerisation and after removal of light they transform to more stable trans-form. Electronic absorption and 1H NMR studies on C1 and C2 revealed rather rapid (1.27 × 10−1 s−1) photoisomerization for C2 relative to C1 (2.7 × 10−2 s−1) which has also been supported by theoretical studies (DFT). The rather fast photoisomerization for C2 compared to C1 may be related to a small energy gap between HOMO and LUMO levels for the respective isomers.


 Please wait while we load your content...
Please wait while we load your content...