Crocin inhibits urea-induced amyloid formation by bovine β-lactoglobulin
Abstract
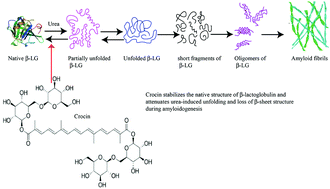
β-Lactoglobulin (β-LG), a major whey protein, is able to form amyloid fibrillar aggregates, when subjected to urea-induced denaturation at pH 7.0. Crocin, being a polar carotenoid, was used to investigate its influence on the urea-induced unfolding of β-LG and formation of amyloid fibrils. Crocin was found to stabilize β-LG structure against urea at pH 7.0 and subsequently inhibited the amyloid fibril formation when challenged with 5 M urea for 2–4 weeks at 37 °C, as observed by thioflavin T fluorescence, congo red binding and transmission electron microscopy. The inhibition by crocin on β-LG amyloid formation in a concentration-dependent manner exhibited a clear correlation between the midpoint of urea denaturation and lag time. Crocin was found to form a complex with β-LG with Kd of 4 × 10−7 M and it could be considered a potential therapeutic agent in the treatment of protein aggregation phenomena.


 Please wait while we load your content...
Please wait while we load your content...