Correlation between the TiO2 encapsulation layer on Pt and its electrochemical behavior†
Abstract
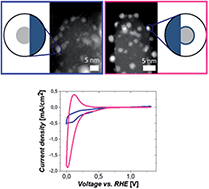
Supported metal catalysts with partial encapsulation resulting from strong metal–support interactions show distinctive structural features which strongly affect their functionalities. Yet, challenges in systematic synthesis and in-depth characterization for such systems limit the present understanding of structure–property relationships. Herein, the synthesis and characterization of two Pt/TiO2 models are conducted by a simple change of the synthesis order, while keeping all other parameters constant. They differ in containing either bare or encapsulated Pt nanoparticles. The presence of an extremely thin and inhomogeneous TiO2 layer is clearly demonstrated on 2–3 nm sized Pt nanoparticles by combination of imaging, energy dispersive X-ray spectroscopy and electron energy loss spectroscopy performed in a transmission electron microscope. The two Pt/TiO2 systems exhibit differences in morphology and local structure which can be correlated with their electrochemical activity and stability using cyclic voltammetry experiments. Beyond enhanced particle stability, we report an increase in H+ intercalation on titania and reduced Pt activity due to partial encapsulation by TiO2. Finally, the growth of an encapsulation layer as a result of cyclic voltammetry measurements is discussed. These results shed light on the in-depth structure–property relationship of catalysts with strong metal–support interactions which leads to enhanced functional materials for electrochromic devices and energy applications.


 Please wait while we load your content...
Please wait while we load your content...