Ion shuttling between emulsion droplets by crown ether modified gold nanoparticles†
Abstract
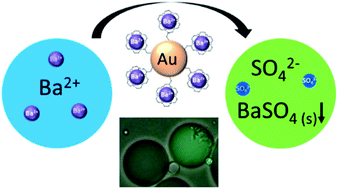
Selective unidirectional transport of barium ions between droplets in a water-in-chloroform emulsion is demonstrated. Gold nanoparticles (GNPs) modified with a thiolated crown ether act as barium ion complexing shuttles that carry the ions from one population of droplets (source) to another (target). This process is driven by a steep barium ion concentration gradient between source and target droplets. The concentration of barium ions in the target droplets is kept low at all times by the precipitation of insoluble barium sulfate. A potential role of electrostatically coupled secondary processes that maintain the electroneutrality of the emulsion droplets is discussed. Charging of the GNP metal cores by electron transfer in the presence of the Fe(II)/Fe(III) redox couple appears to affect the partitioning of the GNPs between the water droplets and the chloroform phase. Processes have been monitored and studied by optical microscopy, Raman spectroscopy, cryogenic scanning electron microscopy (cryo-SEM) and zeta potential. The shuttle action of the GNPs has further been demonstrated electrochemically in a model system.


 Please wait while we load your content...
Please wait while we load your content...