Synthesis and immunological evaluation of N-acyl modified Globo H derivatives as anticancer vaccine candidates†
Abstract
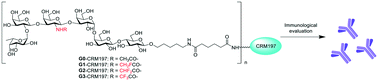
Globo H is a tumor-associated carbohydrate antigen (TACA), which serves as a valuable target for antitumor vaccine or cancer immunotherapies. However, most TACAs are T-cell-independent, and they cannot induce powerful immune response due to their poor immunogenicity. To address this problem, herein, several Globo H analogues with modification on the N-acyl group were prepared through a preactivation-based glycosylation strategy from the non-reducing end to the reducing end. These modified Globo H derivatives were then conjugated with carrier protein CRM197 to form glycoconjugates as anticancer vaccine candidates, which were used in combination with adjuvant glycolipid C34 for immunological studies. The immunological effects of these synthetic vaccine candidates were evaluated on Balb/c mice. The results showed that the fluorine-modified N-acyl Globo H conjugates can induce higher titers of IgG antibodies that can recognize the naturally occurring Globo H antigen on the surface of cancer cells and can eliminate cancer cells in the presence of a complement, indicating the potential of these synthetic glycoconjugates as anticancer vaccine candidates.


 Please wait while we load your content...
Please wait while we load your content...