Modulating β-arrestin 2 recruitment at the δ- and μ-opioid receptors using peptidomimetic ligands†
Abstract
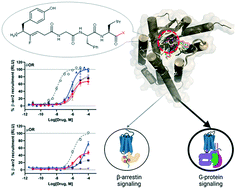
μ-Opioid receptor agonists provide potent and effective acute analgesia; however, their therapeutic window narrows considerably upon repeated administration, such as required for treating chronic pain. In contrast, bifunctional μ/δ opioid agonists, such as the endogenous enkephalins, have potential for treating both acute and chronic pain. However, enkephalins recruit β-arrestins, which correlate with certain adverse effects at μ- and δ-opioid receptors. Herein, we identify the C-terminus of Tyr-ψ[(Z)CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH]-Gly-Leu-enkephalin, a stable enkephalin derivative, as a key site to regulate bias of both δ- and μ-opioid receptors. Using in vitro assays, substitution of the Leu5 carboxylate with amides (NHEt, NMe2, NCyPr) reduced β-arrestin recruitment efficacy through both the δ-opioid and μ-opioid, while retaining affinity and cAMP potency. For this series, computational studies suggest key ligand–receptor interactions that might influence bias. These findings should enable the discovery of a range of tool compounds with previously unexplored biased μ/δ opioid agonist pharmacological profiles.
CH]-Gly-Leu-enkephalin, a stable enkephalin derivative, as a key site to regulate bias of both δ- and μ-opioid receptors. Using in vitro assays, substitution of the Leu5 carboxylate with amides (NHEt, NMe2, NCyPr) reduced β-arrestin recruitment efficacy through both the δ-opioid and μ-opioid, while retaining affinity and cAMP potency. For this series, computational studies suggest key ligand–receptor interactions that might influence bias. These findings should enable the discovery of a range of tool compounds with previously unexplored biased μ/δ opioid agonist pharmacological profiles.


 Please wait while we load your content...
Please wait while we load your content...