Amino alcohol acrylonitriles as broad spectrum and tumour selective cytotoxic agents†
Abstract
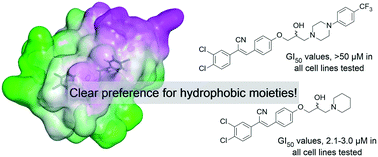
We have identified specific dichlorophenylacrylonitriles as lead compounds in the development of novel anticancer compounds, notably, (Z)-N-(4-(2-cyano-2-(3,4-dichlorophenyl)vinyl)phenyl)acetamide (1) and ANI-7 (2). Herein we specifically probe the SAR associated with the terminal aromatic ring and associated cytoxicity in a broad range of human cancer cell lines. Synthesis of three focused libraries revealed a poor tolerance for electron withdrawing and donating moieties (Library A). A clear preference for hydrophobic substituents on a terminal piperazine moiety (Library B) with good levels of broad spectrum cytotoxicity, e.g.13a (GI50 2.5–6.0 μM), as did the introduction of a methylene spacer with 13i (4-CH3PhCH2; GI50 1.5–4.5 μM). Removal of the aromatic moiety and installation of simple hydrophobic groups (Library C), in particular an adamantyl moiety, afforded highly active broad spectrum cytotoxic agents with GI50 values ranging from 1.7 μM (14k; 1-adamantyl) to 5.6 μM (14i; pyrrolidine). Within these libraries we note lung cancer selectivity, relative to normal cells, of 13h (fluoro substituted acrylonitrile, GI50 1.6 μM, 9.3-fold selective); the colorectal selectivity of 14h (methylpiperidine analogue, GI50 0.36 μM, 6.9-fold selective) and the breast cancer selectivity of 13f (nitrile substituted acrylonitrile, GI50 2.3–6.0 μM, up to 20-fold selective). The latter was confirmed as a novel AhR ligand and a CYP1A1 activating compound, that likely induces cell death following bioactivation; a phenomenon previously described in breast cancer cell populations.

 Please wait while we load your content...
Please wait while we load your content...