Novel fluorinated ring-fused chlorins as promising PDT agents against melanoma and esophagus cancer†
Abstract
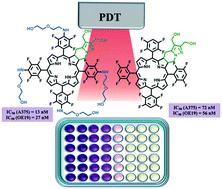
Investigation of novel 4,5,6,7-tetrahydropyrazolo[1,5-a]pyridine-fused chlorins, derived from 5,10,15,20-tetrakis(pentafluorophenyl)porphyrin, as PDT agents against melanoma and esophagus cancer is disclosed. Diol and diester fluorinated ring-fused chlorins, including derivatives with 2-(2-hydroxyethoxy)ethanamino groups at the phenyl rings, were obtained via a two-step methodology, combining SNAr and [8π + 2π] cycloaddition reactions. The short-chain PEG groups at the para-position of the phenyl rings together with the diol moiety at the fused pyrazole ring promote a red-shift of the Soret band, a decrease of the fluorescence quantum yield and an increase of the singlet oxygen formation quantum yield, improving the photophysical characteristics required to act as a photosensitizer. Introduction of these hydrophilic groups also improves the incorporation of the sensitizers by the cells reaching cellular uptake values of nearly 50% of the initial dose. The rational design led to a photosensitizer with impressive IC50 values, 13 and 27 nM against human melanoma and esophageal carcinoma cell lines, respectively.

 Please wait while we load your content...
Please wait while we load your content...