Structural modifications that increase gut restriction of bile acid derivatives†
Abstract
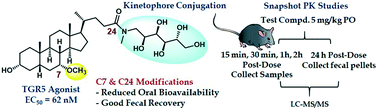
Bile acid derivatives have been investigated as possible therapeutics for a wide array of conditions, including several for which gut-restricted analogs would likely be preferred. These include the prevention of Clostridioides difficile infection (CDI) and the treatment of inflammatory bowel disease (IBD). The design of gut-restricted bile acid analogs, however, is complicated by the highly efficient enterohepatic circulation system that typically reabsorbs these compounds from the digestive tract for subsequent return to the liver. Herein, we report that incorporation of a sulfate group at the 7-position of the bile acid scaffold reduces oral bioavailability and increases fecal recovery in two pairs of compounds designed to inhibit the germination of C. difficile spores. A different approach was necessary for designing gut-restricted bile acid-based TGR5 agonists for the treatment of IBD, as the incorporation of a 7-sulfate group reduces activity at this receptor. Instead, building on our previous discovery that incorporation of a 7-methoxy group into chenodeoxycholic acid derivatives greatly increases their TGR5 receptor potency, we determined that an N-methyl-D-glucamine group could be conjugated to the scaffold to obtain a compound with an excellent mix of potency at the TGR5 receptor, low oral exposure, and good fecal recovery.


 Please wait while we load your content...
Please wait while we load your content...