Using NMR to identify binding regions for N and C-terminal Hsp90 inhibitors using Hsp90 domains†
Abstract
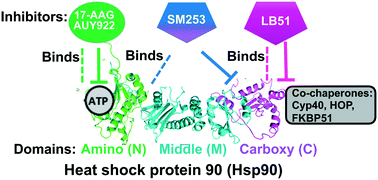
We present the first NMR study of the interaction between heat shock protein 90 (Hsp90) and amino (N)-terminal inhibitors 17-AAG, and AUY922, and carboxy (C)-terminal modulators SM253, and LB51. We show that the two ATP mimics, 17-AAG and AUY922, bind deeply within the ATP binding pocket of the N-terminal domain, consistent with the crystal structures. In contrast, SM253, a C-terminal Hsp90 modulator, binds to the linker region between the N and middle domains. We also show that C-terminal inhibitor LB51 binds to the C-terminus with a more significant spectroscopic change than previously reported using NMR binding studies of C-terminal inhibitors novobiocin and silybin. These data provide key insights into how the allosteric inhibitor SM253 controls the C-terminal co-chaperones and confirms the binding domain of LB51.


 Please wait while we load your content...
Please wait while we load your content...