Identification of 3-hydroxy-1,2-dimethylpyridine-4(1H)-thione as a metal-binding motif for the inhibition of botulinum neurotoxin A†
Abstract
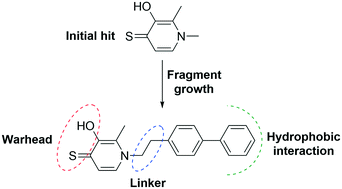
Botulinum neurotoxin serotype A (BoNT/A) is an important therapeutic target owing to its extremely potent nature, but also has potential use as a biowarfare agent. Currently, no therapeutic exists to reverse the long-lasting paralysis caused by BoNT/A. Herein, we describe the identification of 3-hydroxy-1,2-dimethylpyridine-4(1H)-thione (3,4-HOPTO) as a metal binding warhead for the inhibition of BoNT/A1. An initial screen of 96 metal binding fragments identified three derivatives containing the 3,4-HOPTO scaffold to inhibit the BoNT/A1 light chain (LC) at >95% at 1 mM. Additional screening of a 3,4-HOPTO sub-library identified structure–activity relationships (SARs) between N-substituted 3,4-HOPTO derivatives and the BoNT/A1 LC. Subsequent synthesis was conducted to improve on inhibitory potency – achieving low μM biochemical IC50 values. Representative compounds were evaluated in a cellular-based assay and showed promising μM activity.


 Please wait while we load your content...
Please wait while we load your content...