Deacylcortivazol-like pyrazole regioisomers reveal a more accommodating expanded binding pocket for the glucocorticoid receptor†
Abstract
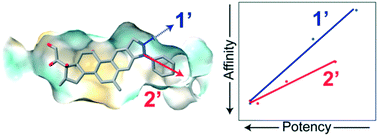
Glucocorticoids (GCs) are widely used, potent anti-inflammatory and chemotherapeutic drugs. They work by binding to the glucocorticoid receptor (GR), a ligand-activated transcription factor, inducing translocation to the nucleus and regulation of genes that influence a variety of cellular activities. Despite being effective for a broad number of conditions, GC use is limited by severe side effects. To identify ligands that are more selective, we synthesized pairs of regioisomers in the pyrazole ring that probe the expanded binding pocket of GR opened by deacylcortivazol (DAC). Using an Ullmann-type reaction, a deacylcortivazol-like (DAC-like) backbone was modified with five pendant groups at the 1′- and 2′-positions of the pyrazole ring, yielding 9 ligands. Most of the compounds were cytotoxic to leukemia cells, and all required GR expression. Both aliphatic and other aromatic groups substituted at the 2′-position produced ligands with GC activity, with phenyl and 4-fluorophenyl substitutions exhibiting high cellular affinity for the receptor and >5× greater potency than dexamethasone, a commonly used strong GC. Surprisingly, phenyl substitution at the 1′-position produced a high-affinity ligand with ∼10× greater potency than dexamethasone, despite little apparent room in the expanded binding pocket to accommodate 1′-modifications. Other 1′-modifications, however, were markedly less potent. The potency of the 2′-substituted and 1′-substituted DAC-like compounds tracked linearly with cellular affinity but had different slopes, suggesting a different mode of interaction with GR. These data provide evidence that the expanded binding pocket opened by deacylcortivazol is more accommodating that expected, allowing development of new, and possibly selective, GCs by substitution within the pyrazole ring.


 Please wait while we load your content...
Please wait while we load your content...