Effect of bulky 2,6-bis(spirocyclohexyl)-substituted piperidine rings in bis(hindered amino)trisulfide on thermal healability of polymethacrylate networks†
Abstract
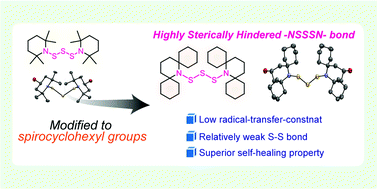
This paper describes the synthesis of highly sterically hindered piperidinyl trisulfide with four spirocyclohexyl moieties, bis(2,6-bis[spirocyclohexyl]piperidine-1-yl)trisulfide (BIBSCPS-S3), from commercially available starting materials in short steps and its application as a dynamic covalent bond for thermally healable polymer networks. Conformational study on the BIBSCPS-S3 moiety in the solid state is performed by single-crystal X-ray diffraction. In bulk, a stress-relaxation experiment reveals that the increase in steric hindrance can not only decrease the activation energy for thermal exchange reactions but also suppress chain-transfer reactions during radical polymerization to some extent. Therefore, the dynamic cross-linking point containing BIBSCPS-S3 moiety can be efficiently incorporated into polymer networks with ethyl, n-butyl, or n-hexyl methacrylate monomers, which is in good accordance with the relatively low chain-transfer constants of the BIBSCPS-S3 moiety determined by the Mayo equation. As a result, BIBSCPS-S3-cross-linked poly(n-hexyl methacrylate) exhibits nearly quantitative damage healability only by simple hot pressing at 90 °C under mild pressure for 24 h.


 Please wait while we load your content...
Please wait while we load your content...